land adaptations to phosphorus deficiency
Cluster roots, an adaptation for growth on the most heavily leached soils in the world
Cluster root development and function
Mycorrhizal symbioses
The most prevalent evolutionary adaptation by land plants (more than 80% of all species) for acquiring P is through mycorrhizal symbioses. For an overview of the mycorrhizal symbiosis, visit http://dmsylvia.ifas.ufl.edu/mycorrhiza.htm.
Cluster roots, an adaptation for growth on the most heavily leached soils in the world
Proteaceae, native species of Western Australia and South Africa, are adapted to grow on the most heavily leached soils in the world (Pate et al., 2001). Species of these regions have evolved specialized structures, cluster roots, also known as proteoid roots, for nutrient uptake from impoverished soils. Beside mycorrhizal associations, cluster roots are regarded as one of the major adaptations for P acquisition. Plant species that form cluster roots usually do not form mycorrhizal associations (Skene, 1998). Cluster roots are formed in most members of the Proteaceae and in several other plant species adapted to habitats of extremely low soil fertility. In native habitats, many plant species that form cluster roots are slow-growing, sclerophyllous shrubs that grow on severely P-deficient soils, such as highly leached sands, sandstones and laterites. The cluster roots of Proteaceae occur in close association with the decomposing litter. Banksia, a genus including trees and shrubs that are dominant in the northern sandplains of south-west Western Australia forms dense mats of cluster roots beneath the litter layer. Growth of these cluster roots is seasonal, starting after the onset of winter rain for nutrient uptake from the newly acquired litter during winter and spring. This period of nutrient uptake is accompanied by nutrient storage in the trunk and leaves. It is followed by senescence of root clusters when the soil surface dries out during the summer.
Cluster root development and function
Adaptation to P-deficiency via formation of cluster roots is the result of a highly coordinated modification of root development and biochemistry. Cluster roots can comprise single clusters of very densely packed determinate lateral rootlets formed on a parent axis, as found broadly similar in such Proteacea as Hakeae spp., Leucadendron laureolum, Grevillea robusta as well as in the legume Lupinus albus. Banksia species, however, form more complex compound clusters . Several features of proteoid root development and morphology are distinguished from that of typical dicot lateral roots. First, lateral roots are randomly initiated from the pericycle of primary roots near the zone of metaxylem differentiation Charlton, 1996), while proteoid roots are initiated in waves. Second, lateral roots are initiated singularly opposite a protoxylem point, in contrast to proteoid roots which are in multiples opposite every protoxylem point within the wave of differentiation. Third, in typical lateral roots, root hair development is highly regulated and occurs from a discrete number of epidermal cells (Ridge, 1995; Malamy & Benfey, 1997; Dolan, 2001), while cluster roots produce a superabundance of root hairs. The accompanying increase in root hair density of clustered rootlets results in an increase of surface area of greater than 100-fold as compared to normal roots. Lastly, contrasting with the indeterminate growth of lateral roots, proteoid root growth is determinate, ceasing shortly after emergence (Fig. 1). This highly synchronous developmental pattern indicates that proteoid root formation is a finely tuned process. Moreover, because root pericycle cells are arrested in the G2 phase of the cell cycle (Skene, 1998, 2000), proteoid root initiation must involve concerted release of multiple pericycle cells from the G2 phase in a wave-like pattern along second order lateral roots.
While mycorrhizal hyphae increase the soil volume that is exploited by roots, cluster roots explore a comparatively small soil volume. The hairy and densely packed lateral rootlets bind tightly to trapped sand and particles of organic matter. The dense aggregates of cluster roots are thought to increase the chance of root exudates to mobilize sparingly soluble inorganic phosphates. Excretion of organic acids and acid phosphatase from cluster roots has been shown for species of the Proteacea. Organic chelators such as organic acids are known to solubilize inorganic bound phosphate. Detailed studies of the carboxylate exudation in Banksia grandis showed exudation of significant amounts of a range of carboxylates when plants were grown on Fe-phosphate or Al-phosphate. Interestingly, different carboxylate patterns were recovered from the rhizosphere, comparing the Fe-phosphate and the Al-phosphate treatments, indicating that the plants perceived a difference in chemistry of their rhizosphere environment. Development of cluster roots was suppressed when plants were grown with higher phosphate supply (K-phosphate).
references
- Dinkelaker B, Hengeler C, Marschner H. 1995. Distribution and function of proteoid roots and other root clusters. Botanica Acta 108: 169-276.
- Gardner W, Parbery D, Barber D. 1981. Proteoid root morphology and function in Lupinus albus. Plant and Soil 60: 143-147.
- Gardner WK, Barber DA, Parbery DG. 1983. The acquisition of phosphorus by Lupinus albus L. III. The probable mechanism by which phosphorus movement in the soil/root interface is enhanced. Plant and Soil 70: 107-124.
- Gerke J, Roemer W, Jungk A. 1994. The excretion of citric and malic acid by proteoid roots of Lupinus albus L.: effect on soil solution concentrations of phosphate, iron, and aluminum in the proteoid rhizosphere in samples of an oxisol and a lurizol. Z. Pflanzenern. Bodenk. 157: 289-294.
- Grierson P, Attiwill P. 1989. Chemical characteristics of the proteoid root mat of Banksia integrifolia L. Australian Journal of Botany 37: 137-143.
- Grierson P. 1992. Organic acids in the rhizosphere of Banksia integrifolia L. f. Plant and Soil 144: 259-265.
- Jeschke W, Pate J. 1995. Mineral nutrition and transport in xylem and phloem of Banksia prionotes (Proteacea), a tree with dimorphic root morphology. Journal of Experimental Botany 46: 895-905.
- Lambers H, Juniper D, Cawthray G, Veneklaas E, Martinez-Ferri E. 2002. The pattern of carboxylate exudation in Banksia grandis (Proteaceae) is affected by the form of phosphate added to the soil. Plant and Soil 238: 111-122.
- Lamont BB. 1973. Factors affecting the distribution of proteoid roots within the root system of two Hakea species. Australian Journal of Botany 20: 155-174.
- Lamont BB. 1982. Mechanisms for enhancing nutrient uptake in plants, with particular reference to mediterranean South Africa and Australia. Botanical Reviews 48: 597-689.
- Lamont BB. 1993. Why are hairy root clusters so abundant in the most nutrient-impoverished soils of Australia? Plant and Soil 155/156: 269-272.
- Pate J, Verboom W, Galloway P. 2001. Co-occurrence of Proteacea, laterite and related oligotrophic soils: coincidental associations or causative inter-relationships? Australian Journal of Botany 49: 529-560.
- Pate J, Watt M (2001) Roots of Banksia spp. (Proteacea) with special reference to functioning of their specialized root clusters. In Waisel Y, Eshel A, Kafkafi U, eds, Plant roots: the hidden half, Ed 3rd. Marcel Dekker Inc., New York
- Skene KR, Kierans M, Sprent J, Raven JA. 1996. Structural aspects of cluster root development and their possible significance for nutrient acquisition in Grevillea robusta (Proteacea). Annals of Botany 77: 443-451.
- Skene KR. 1998b. Cluster roots: some ecological considerations. J. Ecol. 86: 1060-1064.
- Skene KR. 2000. Pattern Formation in Cluster Roots: Some developmental and evolutionary considerations. Annals of Botany 85: 901-908.
- Torrey JG (1986) Endogenous and exogenous influences in the regulation of lateral root formation. In Jackson AB, ed, New Root Formation in Plants and Cuttings. Martinus Nijhoff, Dordrecht
- Uhde-Stone C, Li A, Daemen MJ, Allan DL, Vance CP (2001) Isolation and characterization of white lupin proteoid root expressed sequence tags (ESTs) associated with plant hormones. In Horst WJea, ed, Plant Nutrition. Food Security and Sustainability of Agro-ecosystems through Basic and Applied Research, Vol 92. Kluwer, Dordrecht, pp 32-33



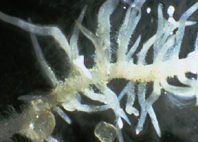 Fig 1. proteoid root of white lupin, closeup
Fig 1. proteoid root of white lupin, closeup